[December 2015] Japanese Supreme Court (SC) Decision on Patent Term Extension (PTE) Application
2015.12
SAEGUSA IP UPDATE
1.Summary of Decision
On November 17, 2015, the SC made a significant decision regarding a PTE application for pharmaceutical-related inventions.
The issue at stake was the availability of a PTE based on an additional marketing authorization obtained following a change in the dosage regimen of the patented product, even if the ingredients, effect, and efficacy thereof were identical to those of the medicament covered by a prior marketing authorization.
The SC concluded that a PTE should be allowed with regard to this particular case, and clarified some of the requirements for such a PTE, which had been unclear from previous case law. In accordance with the SC decision, the JPO is now revising the PTE Examination Guidelines.
2.Background
In 2003, the U.S. company Genentech Inc., obtained a patent for a composition for treating cancer, the composition comprising an effective amount of vascular endothelial cell growth factor antagonists. In 2007, Genentech obtained a marketing authorization (1st MA) for “Avastin (Bevacizumab),” an anti-cancer preparation covered by the patent, from the Ministry of Health, Labour and Welfare.
According to the Japanese patent system, the duration of the patent right (20 years from the filing date) may be extended for up to 5 years with respect to pharmaceutical-related patents, if there was a period during which a patented invention could not be worked due to procedures for obtaining marketing authorization.
Upon obtaining the 1st MA, Genentech filed the 1st PTE application seeking an extension of roughly 4 years. The JPO granted the extension.
In 2009, after an additional marketing authorization for a different dosage regimen (2nd MA) was obtained, Genentech filed the 2nd PTE application seeking an extension of 5 years.
However, the JPO rejected the 2nd PTE application due to the existence of the 1st MA. Genentech appealed, seeking a revocation of the JPO’s decision.
3.Issue
According to Article 67-3(1)(i) of the Patent Act, a PTE application filed based on a marketing authorization will be rejected unless the marketing authorization is deemed to have been necessary for the working of the patented invention.
In the subject case, a marketing authorization (1st MA) was already granted with respect to the same patented invention, prior to the marketing authorization (2nd MA) on whose basis the PTE application in question was filed. Therefore, the issue was whether the PTE application in question should be rejected on the basis of the existence of the 1st MA.
The dosage regimen of the medicament covered by the 2nd MA is as follows:
The “active ingredient Bevacizumab” is usually “administered at a dose of 7.5 mg/kg (body weight)
to an adult in the form of an intravenous drip injection, in combination with other anti-cancer agents,
at a dosage interval of more than three weeks.”
Prior to the 2nd MA, the 1st MA was obtained for a medicament identical to the subject medicament of the 2nd MA, except for a different dosage regimen.
The dosage regimen of the medicament covered by the 1st MA is as follows:
The “active ingredient Bevacizumab” is usually “administered at a dose of 5 mg/kg (body weight)
or 10 mg/kg (body weight) to an adult in the form of an intravenous drip injection, in combination
with other anti-cancer agents, at a dosage interval of more than two weeks.”
Diagram showing the differences between the granted claim scope, and the two marketing authorizations in question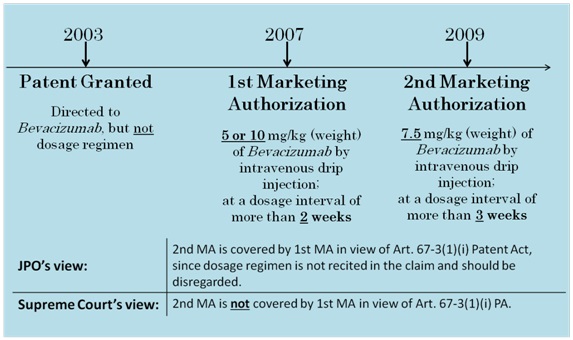
The JPO concluded, based on Examination Guidelines, that the “working of the patented invention” in Article 67-3(1)(i) of the Patent Act should be construed as the working of a medicament specified by the claimed features only.
More specifically, the JPO concluded that although the dosage regimen was included in the definition of the approved medicament in the 2nd MA, this matter should be disregarded since these were not claimed features.
The JPO then took the position that the “patented invention” already became workable following the 1st MA. Accordingly, the JPO rejected the subject PTE application, stating that it was unnecessary to obtain the 2nd MA for the “working of the patented invention”.
4.Holding of SC
The SC reversed the JPO’s position, and indicated that whether the 2nd MA is necessary for the working of the patented invention should be considered based on a comparison between the 1st MA and the 2nd MA. The SC further stated that the comparison should be made based on the elements examined that are directly relevant to the substantial identicalness as a medicament, i.e., in terms of the ingredients, quantity (i.e., amount of the active ingredients), dosage regimen, effect and efficacy of the medicament in question.
Noting that the production and sale of the subject medicament for use in a combination therapy of XELOX treatment (1 cycle = 3 weeks, only a 2-hour infusion and administration of an oral medicine alone are required) with Bevacizumab treatment did not become available following the 1st MA, the SC concluded that the 2nd MA allowed the combination therapy for the first time, and that the reason for rejection under Article 67-3(1)(i) could therefore not be applied to the 2nd MA.
5.JPO Activity Following SC Decision
The SC decision made it necessary for the JPO to revise the PTE Examination Guidelines. Currently, the JPO is considering a revision of the Examination Guidelines for Patent and Utility Model, Part IX – Extension of Patent Term, and expects to release the revised Examination Guidelines in the spring of 2016.
The JPO announced that examination of PTE applications filed based on an additional marketing authorization granted following a prior marketing authorization would, in principle, be suspended until the publication of the revised Examination Guidelines.
According to the SC ruling, a PTE may be granted based on each marketing authorization of a medicament if any of the ingredients, quantity, dosage regimen, effect and efficacy thereof differs from those of a previously approved product. If the Examination Guidelines are revised in accordance with this ruling, increased patent protection for medicaments etc., having a new dosage regimen is expected to be provided.


